Celeste Kinglaw
Legacy of Excellence in Clinical Trials
With a relentless commitment to innovation, patient safety, and ethical standards, we provide groundbreaking clinical trials that shape the future of healthcare.
About
Celeste Kinglaw
Welcome to Celeste Kinglaw, where compassion meets innovation in the pursuit of medical excellence. Established with a vision to advance healthcare through transformative clinical research, our center has become a trusted name in the industry. With a focus on safety, integrity, and patient-centered care, we are dedicated to conducting clinical trials that pave the way for groundbreaking medical treatments and therapies.
Celeste Kinglaw
Clinical Key Areas
Bespoke Medical Expertise Centered on Your Unique Journey
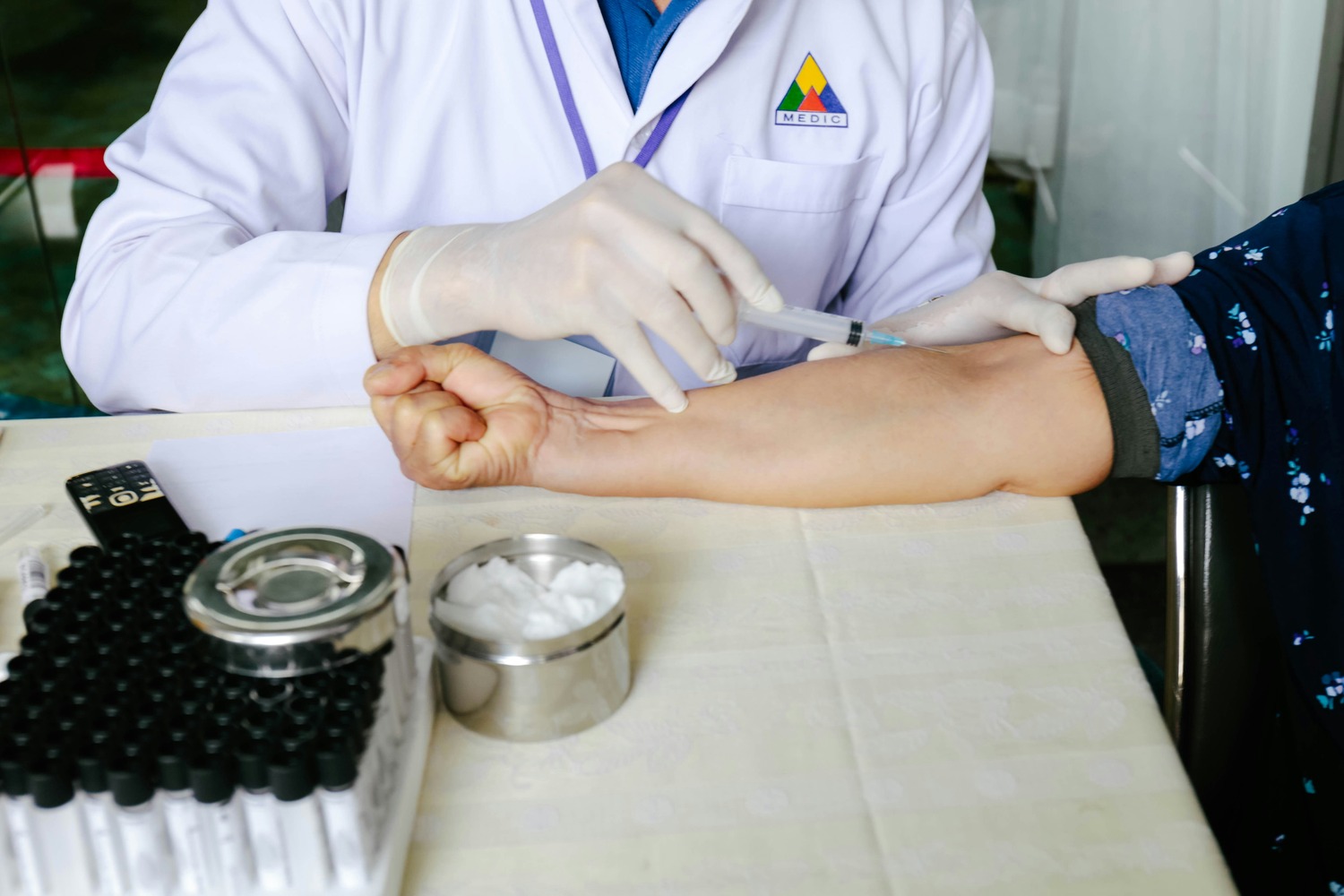
Early-Phase Clinical Trials
We specialize in Phase I and II clinical trials, focusing on evaluating the safety, dosage, and efficacy of new treatments. With state-of-the-art facilities and expert oversight, we ensure these initial studies are conducted with precision and care.

Adaptive Clinical Trial Design
Our team utilizes adaptive trial methodologies, enabling real-time adjustments to protocols based on interim results. This approach enhances trial efficiency, reduces costs, and accelerates the development of promising therapies.

Rare Disease Research Solutions
Celeste Kinglaw is committed to addressing underserved conditions. We design and execute clinical trials for rare and complex diseases, offering hope to patients and families who need it most this research solutions.

Decentralized Clinical Trials (DCTs)
To increase accessibility, we offer decentralized trials that combine virtual and in-person components. Participants can engage from the comfort of their homes while still receiving exceptional care and monitoring.
Why Choose Us
Our Approach to Research Excellence
Collaboration and Partnership
We work closely with pharmaceutical companies, biotech firms, and academic institutions to ensure that our research drives impactful results. By fostering partnerships, we accelerate the development of innovative therapies.
Technology-Driven Insights
Our research is powered by the latest advancements in medical technology, including artificial intelligence and data analytics. These tools allow us to analyze complex datasets with precision, uncovering trends and solutions that were previously unattainable.
Regulatory Compliance and Integrity
Every study at Celeste Kinglaw adheres to the highest standards of regulatory compliance and ethical conduct. Our rigorous protocols ensure accuracy, transparency, and trustworthiness in all research endeavors.
Celeste Kinglaw
Patient Testimonials
- Amy R.
Participating in a trial at Celeste Kinglaw was one of the best decisions I’ve ever made. The staff was incredibly caring and walked me through every step of the process. I felt safe and valued throughout my journey.
Read more - Michael P.
Working with Celeste Kinglaw has been a game-changer for our research collaborations. Their team’s professionalism, attention to detail, and dedication to excellence are unmatched in the industry.
Read more - Kris L.
The team at Celeste Kinglaw goes above and beyond to ensure that patients are informed, comfortable, and respected. Their patient-first approach truly sets them apart.
Read more - Carlos J.
I was nervous about joining a clinical trial, but the compassionate team at Celeste Kinglaw made the entire experience seamless. Their expertise and care made all the difference.
Read more - Mona E.
As a caregiver, I was amazed by the support Celeste Kinglaw provided to my loved one. They treated us like family and made sure we had all the resources and answers we needed.
Read more - Mark B.
Celeste Kinglaw delivers consistent, high-quality results. Their commitment to scientity and participant safety ensures every project is successful from start to finish.
Read more
Connect with Celeste Kinglaw
Address
155 Neil Bay Dr Friday Harbor, WA 98250 United States
Email Address
Phone Number
833-928-1398
